Homogeneous and Bio-Inspired Catalysis.
Due to their molecularly defined architecture, homogeneous catalysts enable the conversion of chemical entities in to useful products with ultimate precision, which explains their use in organic synthesis and chemical manufacturing. Current challenges in the field of homogeneous catalysis include both the search for new catalytic reactions that make use of predesigned functional groups to a much lesser extent and the development of more sustainable catalysts, e.g. non-noble metal catalysts. In search of suitable solutions to these challenges, nature’s catalysts offer a wealth of inspiration and are used as designer mould for new synthetic catalysts. Metallo-enzymes are of particular interest in this sense, as many of these enzymes catalyze reactions that are unprecedented in synthetic chemistry and typically use first row transition metals in their active site instead of the heavier metals frequently used in man-made homogeneous catalysis.
Research topics:
• Bioinorganic Chemistry
• Oxidation Catalysis
• Catalytic Biomass Conversion
• Catalyst recycling / hybrid catalysts
• Miscellaneous projects
To Klein Gebbink profile page (opens in a new window/tab)
A general interest lies in the role of transition metal ions in biology, in particular in the catalytic role of these metal ions in metalloenzymes. The class of mono-nuclear non-heme iron enzymes comprising a so-called 2-His-1-carboxylate facial triad in their active site has attracted considerable attention in the past decades. Whereas these enzymes share the same active site structural motif, they catalyze an astonishing wide variety of different (oxidative) transformations. In trying to understand and grasp the reactivity of these enzymes, iron complexes derived from tripodal, tridentate, mono-ionic bis(1-alkylimidazol-2-yl)propionate ligands are studied. These studies have shown the accurate structural resemblance of synthetic Fe-complexes derived from these ligands with the enzyme active site and provide further insight in enzyme reactivity.
Highlights:

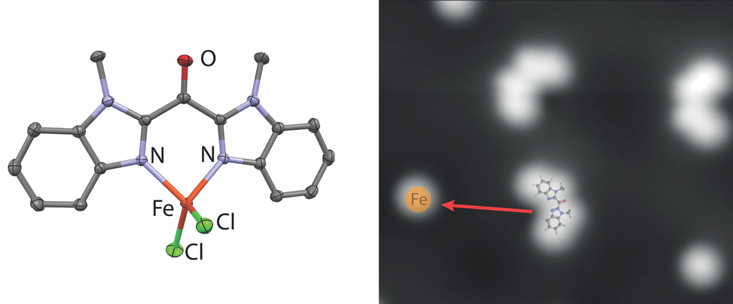
Emma Folkertsma, Joost van der Lit, Francesca Di Cicco, Martin Lutz, Robertus J. M. Klein Gebbink, Ingmar Swart, and Marc-Etienne Moret.
Combination of Scanning Probe Microscopy and Coordination Chemistry: Structural and Electronic Study of Bis(methylbenzimidazolyl)ketone and Its Iron Complex.
ACS Omega, 2017, 2, 1372-1379.
DOI: 10.1021/acsomega.6b00510
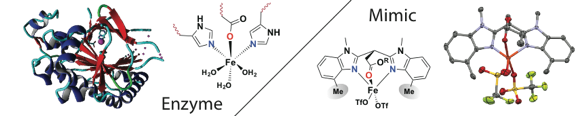
Emma Folkertsma, Esther F. de Waard, Gerda Korpershoek, Arnoldus J. van Schaik, Naiara Solozabal Mirón, Mandy Borrmann, Sjoerd Nijsse, Marcel A. H. Moelands, Martin Lutz, Matthias Otte, Marc-Etienne Moret and Robertus J. M. Klein Gebbink.
Mimicry of the 2-His-1-Carboxylate Facial Triad Using Bulky N,N,O-Ligands: Non-Heme Iron Complexes Featuring a Single Facial Ligand and Easily Exchangeable Co-Ligands.
Eur. J. Inorg. Chem., 2016, 1319–1332.
DOI: 10.1002/ejic.201501406
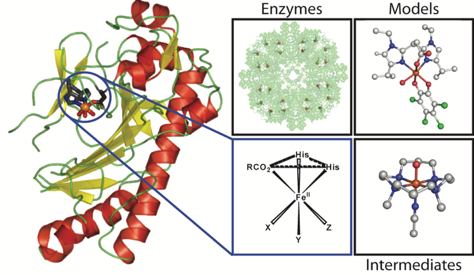
Pieter C. A. Bruijnincx, Gerard van Koten, and Robertus J. M. Klein Gebbink.
Mononuclear Non-Heme Iron Enzymes with the 2-His-1-Carboxylate Facial Triad: Recent Developments in Enzymology and Modeling Studies.
Chem. Soc. Rev., 2008, 37, 2716-2744.
DOI: 10.1039/b707179p
Inspired by non-heme enzymes, the chemistry of non-heme coordination complexes of iron and manganese are studied with a special emphasis on their catalytic properties. Through the design of open-chain N4-ligands, the use of such complexes is explored in C–H bond activation and oxidation, and in the oxidative conversion of C=C double bonds to epoxides and diols. Next to optimizing the activity and selectivity in catalysis, particular emphasis in these studies is on the development of practical oxidation catalysts with increased lifetimes. The catalysts that are developed are explored in the conversion of synthetic organic molecules as well as terpenes and fatty acids (biomass conversion).
Highlights:
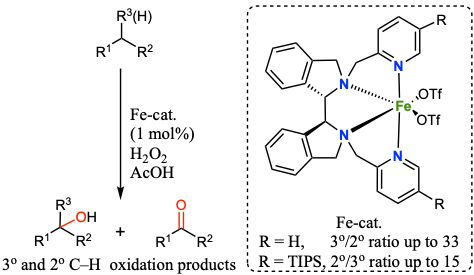
Jianming Chen, Martin Lutz, Michela Milan, Miquel Costas, Matthias Otte and Robertus J. M. Klein Gebbink.
Non-Heme Iron Catalysts with a Rigid Bis-Isoindoline Backbone and Their Use in Selective Aliphatic C–H Oxidation.
Advanced Synthesis & Catalysis, 2017, 359, 2590-2595.
DOI: 10.1002/adsc.201700239
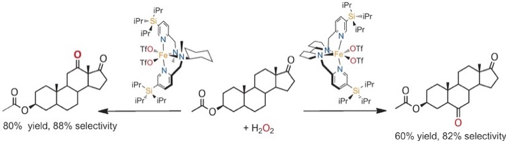
David Font, Merce Canta, Michela Milan, Olaf Cusso, Xavi Ribas, Robertus J. M. Klein Gebbink, and Miquel Costas.
Readily Accessible Bulky Iron Catalysts exhibiting Site Selectivity in the Oxidation of Steroidal Substrates.
Angew. Chem. Int. Ed., 2016, 55, 5776-5779.
DOI: 10.1002/anie.201600785
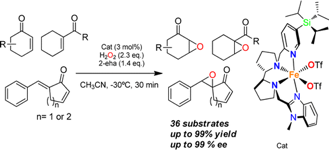
Olaf Cussó, Marco Cianfanelli, Xavi Ribas, Robertus J. M. Klein Gebbink and Miquel Costas.
Iron Catalyzed Highly Enantioselective Epoxidation of Cyclic Aliphatic Enones with Aqueous H2O2.
J. Am. Chem. Soc., 2016, 138, 2732-2738.
DOI: 10.1021/jacs.5b12681
Due to the anticipated depletion of fossil feedstocks, the search for alternative and renewable chemical feedstocks receives a lot of attention. Biomass is such a potential resource for the sustainable production of commodity chemicals and other chemical building blocks. Biomass-derived feedstocks, such as sugars and polyols, are highly oxygenated, mostly in the form of hydroxyl groups. In order to make use of these feedstocks, (partial) deoxygenation is required. Deoxydehydration (DODH) reactions, which constitute a combination of deoxygenation and dehydration, can efficiently convert vicinal diols and polyols into olefins. Molecular catalysts for DODH reactions are developed. Mostly Cp-based rhenium complexes are studied, whereas alternative metals are also considered. Other projects involved in catalytic biomass conversion focus on the oxidation of unsaturated fatty acids and plant oils.
Highlights:
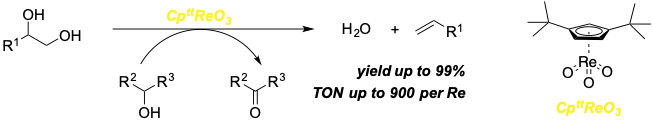
Jing Li, Martin Lutz, Matthias Otte, and Robertus J. M. Klein Gebbink.
A Cptt-Based Trioxo-Rhenium Catalyst for the Deoxydehydration of Diols and Polyols.
ChemCatChem, 2018, 10, 4755-4760.
DOI: 10.1002/cctc.201801151

Suresh Raju, Marc-Etienne Moret, and Robertus J. M. Klein Gebbink.
Rhenium-Catalyzed Dehydration and Deoxydehydration of Alcohols and Polyols: Opportunities for the Formation of Olefins from Biomass.
ACS Catal, 2015, 5, 281-300.
DOI: 10.1021/cs501511x
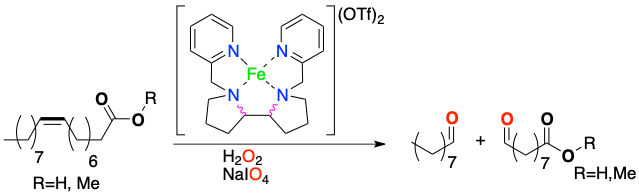
Peter Spannring, Vital Yazerski, Pieter C. A. Bruijnincx, Bert M. Weckhuysen,and Robertus J. M. KleinGebbink.
Fe-Catalyzed One-Pot Oxidative Cleavage of Unsaturated Fatty Acids into Aldehydes with Hydrogen Peroxide and Sodium Periodate.
Chem. Eur. J., 2013, 19, 15012-15018.
DOI: 10.1002/chem.201301371
Catalyst recycling / hybrid catalysts
A number of other projects are explored as well. Mostly, these concern with sustainability aspects of and in catalysis. One of these aspects is the ability to recycle homogeneous catalysts. In addition, coordination complexes are explored in the context of energy-related research, like e.g. catalytic hydrogen production.
Highlights:
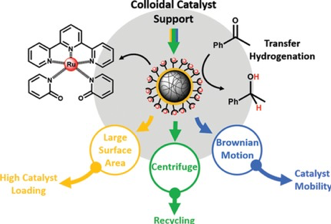
Bas G. P. van Ravensteijn, Dirk-Jan Schild, Willem K. Kegel, and Robertus J. M. Klein Gebbink.
The Immobilization of a Transfer Hydrogenation Catalyst on Colloidal Particles.
ChemCatChem, 2017, 9, 440-450.
DOI: 10.1002/cctc.201601096
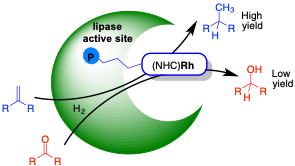
M. Basauri-Molina, C. F. Riemersma, M. A. Wurdemann, H. Kleijn and R. J. M. Klein Gebbink.
Lipase active site covalent anchoring of Rh(NHC) catalysts: towards chemoselective artificial metalloenzymes.
Chem. Commun., 2015, 51, 6792-6795.
DOI: 10.1039/c4cc09700a
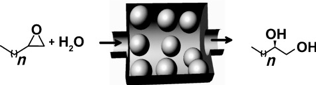
Mozaffar Shakeri, Robertus J. M. Klein Gebbink, Petra E. de Jongh, and Krijn P. de Jong.
Tailoring the Window Sizes to Control the Local Concentration and Activity of (salen)Co Catalysts in Plugged Nanochannels of SBA-15 Materials.
Angew. Chem. Int. Ed., 2013, 52, 10854-10857.
DOI: 10.1002/anie.201304640
Highlights:
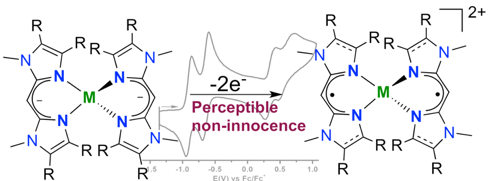
Pradip Ghosh, Richard Naastepad, Charl F. Riemersma, Martin Lutz, Marc-Etienne Moret, and Robertus J. M. Klein Gebbink.
Noninnocent β-Diiminate Ligands: Redox Activity of a Bis(alkylimidazole)methane Ligand in Cobalt and Zinc Complexes.
Chem. Eur. J., 2017, 23, 10732-10737.
DOI: 10.1002/chem.201701215
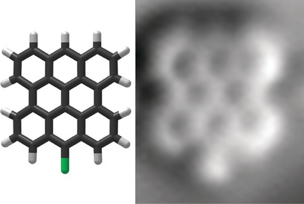
Peter H. Jacobse, Adri van den Hoogenband, Marc-Etienne Moret, Robertus J. M. Klein Gebbink, and Ingmar Swart.
Aryl Radical Geometry Determines Nanographene Formation on Au(111).
Angew. Chem. Int. Ed., 2016, 55, 13052-13055.
DOI: 10.1002/anie.201606440